
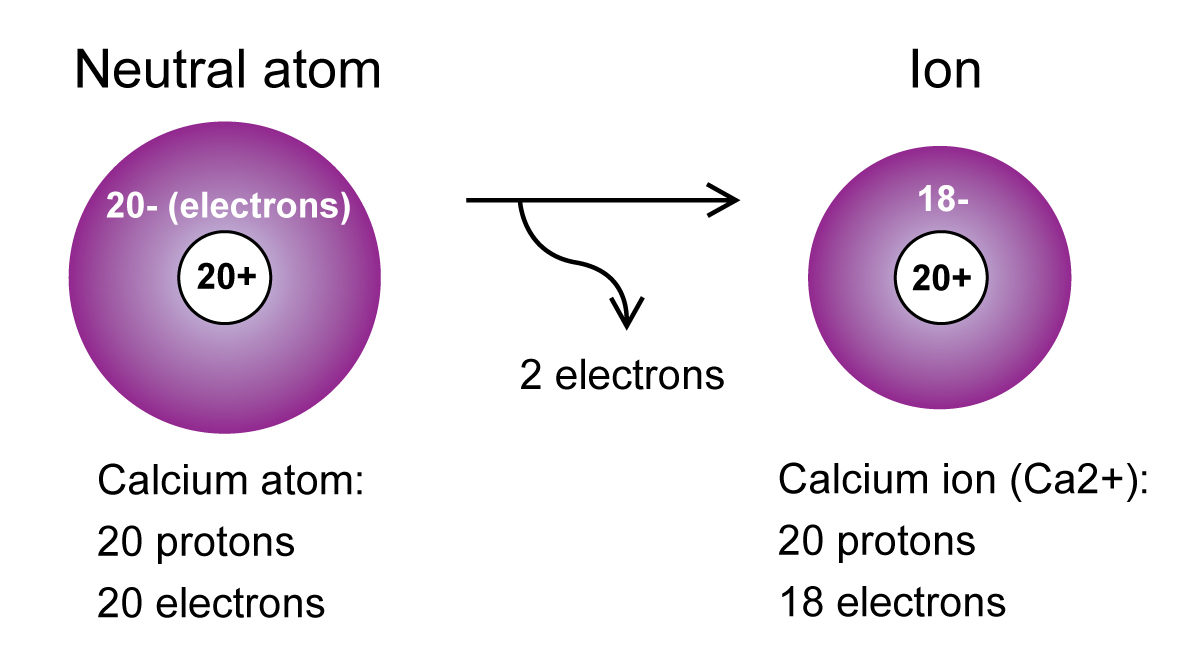

There are 4 protons, 5 neutrons, and 4 electrons. Fill in the rest of the table: Atomic Numberġ. Arrange the following elements in order of increasing (a) number of protons (b) number of neutrons (c) mass.Ģ7Co, when A=59 56Fe, when Z=26 11Na, when A=23 80Br, when Z=35 29Cu, when A=30 55Mn, when Z=25ĥ. Charge -1, 18 electrons, mass number 36.Ĥ.(The periodic table is required to solve these problems) Given the following, identify the subatomic particles present. Identify the subatomic particles (protons, electrons, neutrons, and positrons) present in the following:ģ. Identify the number of protons, electrons, and neutrons in the following atom.Ģ. Note: The atomic mass number is not the same as the atomic mass seen on the periodic table. Charge is written with the number before the positive or negative sign.In ions, # Electrons = # Protons - (Charge).In neutral atoms, # Electrons = # Protons.# Protons = Proton Number or Atomic Number.Proton number(or atomic number) is abbreviated Z.Atomic mass number is abbreviated as A.# Neutrons = Atomic Mass Number - Proton Number.In neutral atoms, the charge is omitted.Ībove is the atomic symbol for helium from the periodic table, with the atomic number, elemental symbol, and mass indicated.Įvery element has a specific number of protons, so the proton number is not always written (as in the second method above). The atomic mass number of Carbon is 12 amu, the proton number is 6, and it has no charge.


 0 kommentar(er)
0 kommentar(er)
